Unwinding of the Public Health Emergency (PHE)
The Pandemic is Ending: Key Dates for Telehealth You Should Know
With the COVID-19 Pandemic coming to a close, it is crucial that telehealth providers pay attention to the various changes coming their way. Check out our PHE timeline to ensure you are up to date on all critical dates.
During the COVID-19 pandemic, telehealth solidified its standing as a critical health care delivery modality. Telehealth has since become a preferred method for health care providers to reach out to patients—especially those in remote or rural areas. Declining rates of COVID-19 cases and the recent Biden Administration announcement that the federal Public Health Emergency (PHE) declaration will be lifted on May 11, 2023, many health care providers are wondering how this will impact telehealth. In this post, we discuss impending changes to telehealth regulations tied to the end of the PHE, how these changes will affect telehealth providers, and potentially alter the future telehealth landscape.
Timeline of Legislation Impacting Telehealth
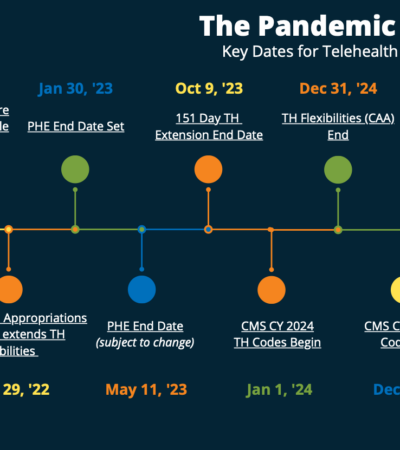
- November 1, 2022: CMS Released the Final ’23 CY Medicare Physician Fee Schedule
- December 29, 2022: The Consolidated Appropriations Act (CAA) is released and extends telehealth flexibilities
- January 30, 2022: The end date for the PHE is set for May
- May 11, 2023: The PHE comes to a close
- October 9, 2023: The 151-day telehealth extensions end
- January 1, 2024: CMS’s CY 2024 telehealth codes go into effect
- December 31, 2024: The flexibilities afforded by the CAA extension end
- December 31, 2024: CMS’s CY 2024 telehealth codes end
- January 1, 2025: CMS’s CY 2024 telehealth codes go into effect
Pending Telehealth Legislation
CTRC is carefully monitoring several key pieces of legislation yet to be finalized that have significant impacts on telehealth. We will continue to provide updates on each of these issues as they develop.
Telemedicine: Prescribing Controlled Substances
During the PHE, the Drug Enforcement Administration (DEA) waived the requirement under the Ryan Haight Act for an in-person exam in order to be prescribed a controlled substance. This ensured that both established and new patients could access medically necessary prescriptions via telemedicine. However, unless the DEA takes action, the in-person requirement will be reinstated when the PHE expires on May 11. Prescribing controlled substances without an in-person exam will be prohibited.
DEA’s Proposed Rules on Telemedicine Controlled Substances Prescribing
In a move to prevent lapses in patient care, on February 24, 2023, the Drug Enforcement Agency (DEA) announced proposed rules for prescribing controlled substances via telemedicine after the COVID-19 Public Health Emergency ends. The DEA’s proposed rules are open for public comment for thirty days prior to issuing final regulations.
Intended to ensure continuity of care under the current telehealth flexibilities, the proposed rules introduce two new options:
- A virtual-first process by which a practitioner can issue an initial prescription for a controlled substance without first performing an in-person patient exam if:
- The medication is a non-narcotic Schedule III, IV, or V controlled substance or buprenorphine for the treatment of OUD
- The prescribed amount, referred to as a “telemedicine prescription,” does not exceed a 30-day supply.
- Before any additional prescriptions can be written, the patient must have an in-person exam.
- A “qualified telemedicine referral” protocol in which a patient has an initial in-person exam with a practitioner who then refers the patient to another practitioner. The second practitioner can conduct a telemedicine patient exam and prescribe a controlled substance without having to examine the patient in person. The telemedicine practitioner can prescribe Schedule II, III, IV, and V, and narcotic controlled substances under this referral process.
If you would like to submit a comment to the DEA regarding the rules, keep in mind that electronic comments must be submitted, and written comments must be postmarked on or before March 31, 2023. Commenters should be aware that the electronic Federal Docket Management System will not accept comments after 11:59 p.m. Eastern Time on the last day of the comment period. Please go to this website and follow the instructions to submit comments.
Telehealth Legislation Terminating at the End of the PHE (May 11, 2023)
Medicare Payment Parity
On March 6, 2020, the Centers for Medicare & Medicaid Services (CMS) initiated higher reimbursement for telehealth services at non-facilities such as a patient’s home. This allowed Medicare to pay for telehealth services as if provided in person, meaning telehealth visits were reimbursed at the same rate as regular in-person office visits.
Unless lawmakers act to extend the policy, parity in reimbursement rates for telehealth services will end on May 11, 2023, with telehealth rates reverting to lower pre-pandemic levels .
End of Telehealth and Remote Patient monitoring (RPM) Copayment Waivers
During the PHE, health care providers who provided telehealth or remote patient monitoring (RPM) services to Medicare beneficiaries were not subject to administrative sanctions under the federal Anti-Kickback Statute or the Civil Monetary Penalty and Exclusion Act for reducing or waiving cost-sharing amounts. After May 11, health care providers may no longer reduce or waive any cost-sharing obligations that patients owe for telehealth or RPM services unless the Office of Inspector General (OIG) issues additional guidance or an extension.
Medicare RPM Services Limited to Established Patients
After the PHE expires, billable Medicare RPM services will be limited to established patients. According to CMS statements, after the PHE ends, the physician must first perform a new patient evaluation and management service before providing RPM to a new patient.
End of HIPAA-Related Enforcement Discretion
For the duration of the PHE, the HHS Office for Civil Rights (OCR) exercised enforcement discretion allowing providers to use telehealth in good faith even if their platforms or software did not follow Health Insurance Portability and Accountability Act (HIPAA) rules. After the PHE ends on May 11, the OCR will resume enforcement of penalties on providers using technologies that are noncompliant with HIPAA rules.
Telehealth Legislation Terminating at the End of 2023
Virtual Direct Supervision
During the PHE, CMS temporarily altered the direct supervision rules to allow the supervising professional to be remote and use real-time, interactive audio-video technology to carry out supervision. CMS refused to extend this temporary policy past the end of the calendar year in which the PHE expires. As a result, unless CMS changes its policy in future rulemaking, virtual direct supervision will expire at the end of 2023.
Telehealth Legislation Expiring at the End of 2024
Temporary Medicare Changes
The Consolidated Appropriations Act (CAA) of 2023 extended several telehealth flexibilities that were authorized during the COVID-19 PHE until December 31, 2024. These flexibilities include:
- Providers who are eligible to bill Medicare may bill for telehealth services irrespective of the location of the provider or patient.
- Audio-only telehealth visits remain reimbursable.
- The list of providers who are eligible to deliver telehealth services has also been expanded to include physical therapists, occupational therapists, speech-language pathologists, and audiologists.
- The acute hospital care at-home program will continue to provide hospital services to patients in their homes, including through telehealth.
- The use of telehealth for recertification of eligibility for hospice care is allowed.
- Patients with High Deductible Health Plans coupled with Health Savings Accounts are permitted to use first-dollar coverage for telehealth services without having to fulfill their minimum deductible requirements.
- Federally Qualified Health Centers (FQHCs) and Rural Health Clinics (RHCs) have been granted the authority to provide telehealth services to Medicare beneficiaries.
- Postponed pre-requisite in-person requirement for mental health services furnished through telehealth until after December 31, 2024.